Class D IVD manufacturers face fast-approaching IVDR transition deadlines, though some are more familiar with the regulatory process. In this article, Dr. Oliver Eikenberg discusses the difference between "new" and "old" Class Ds, and regulatory logistics they need to consider during the transition process.
Under the IVDR, Class D devices now fall into two broad categories: those previously covered under IVDD’s List A and B (e.g., HIV, Hepatitis, ABO, Blood Grouping, Toxoplasma, Cytomegalovirus, Chlamydia, Rubella, PSA, Self-Test for Blood Glucose), and those newly classified under IVDR (e.g., SARS-CoV-2, Malaria, Dengue fever, Chikungunya, Zika virus, Treponema pallidum, Trypanosoma cruzi). Both groups (“old” and “new”) need to comply with provisional transition requirements and consider Notified Body operations.
While the first group benefits from existing regulatory experience and possible legacy status under IVDD (extended EC Certificates issued by Notified Bodies), the second group typically do not have the same level of regulatory experience and feedback from Notified Bodies. Manufacturers of devices newly classified under Class D may feel that they must comply with a lot of new requirements, which might be more challenging than for manufacturers of established Class D devices.
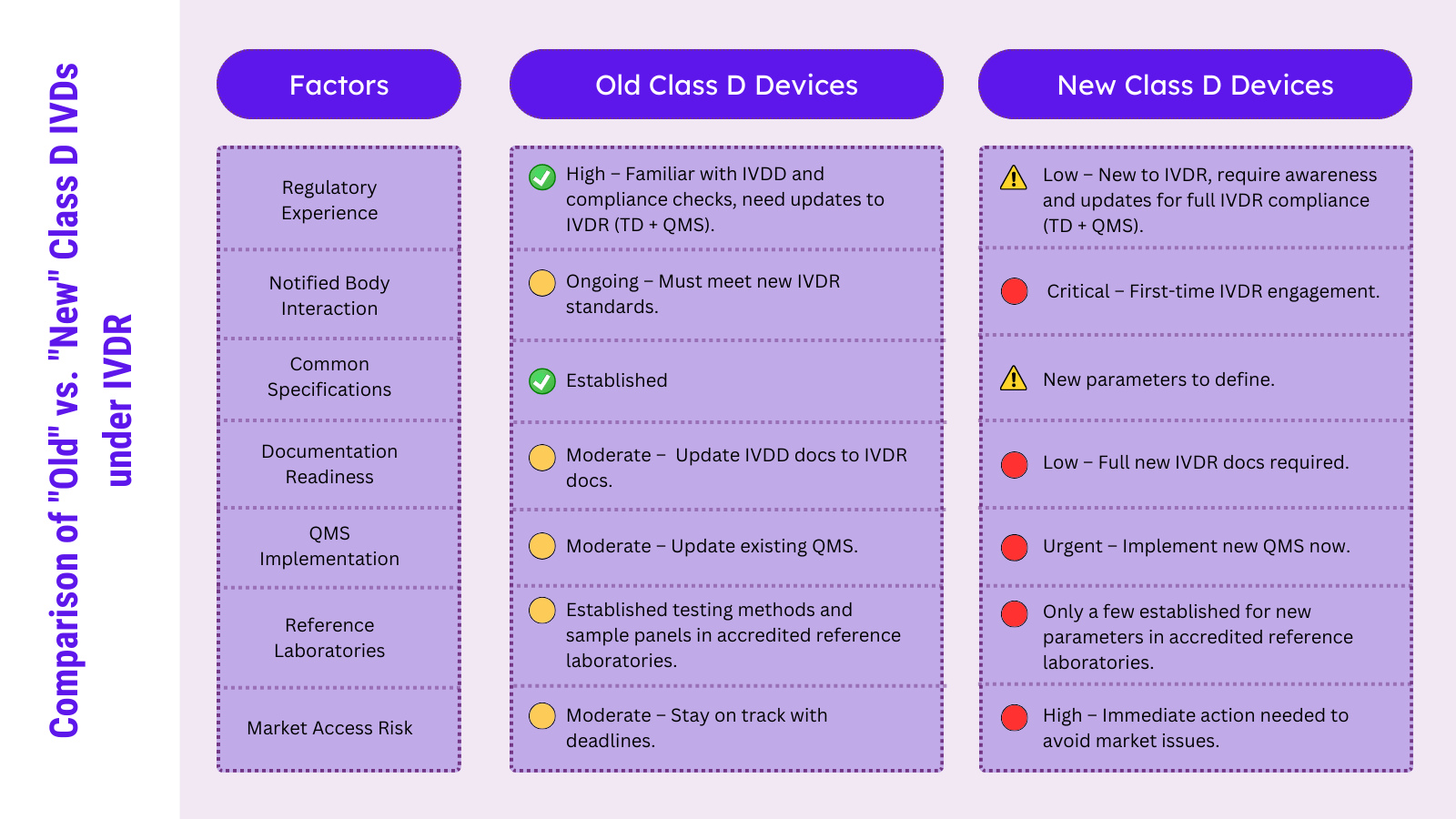
The first category of Class D devices is or soon will be under full Notified Body control. The current EC Certificates extended by Notified Bodies under IVDD as legacy devices will soon end. Even if there are new processes implemented through the IVDR and new EU reference laboratories officially established, the overall process and experience with these Class D parameters should be similar compared to the IVDD. Common specifications are established for most of these Class D parameters, so that EU reference laboratories have long-term experience with the required performance testing. Overall, the main activities for manufacturers of old Class D devices are to implement and update their QMS and Technical Documentation to the obligations introduced by the IVDR.
The second group of IVD devices comprises new analytes and claims that were not addressed under IVDD. A substantial number of these devices might be covered under “general” IVDs according to the IVDD and still be marketed as legacy devices, providing that the transitional obligations of the IVDR are met. These IVD manufacturers might already be in contact with a Notified Body. They will also need to have an independent audit of their Technical Documentation and QMS for the first time. This independent Notified Body feedback may raise a lot of questions and often identifies a different understanding of adequate and objective documentation and interpretation of IVDR obligations between the manufacturer and Notified Body.
Further, the common specifications and EU reference laboratories have only been established for a few parameters (e.g., (EU) 2022/1107 for Treponema pallidum, Trypanosoma cruzi, SARS-CoV-2). Additional parameters may follow in the next month, but it can be expected that the work and questions from all parties involved in this new process will be more time-consuming than for established markers. For this reason, IVD manufacturers in this group need to act now to prepare for this change and stay on the EU market.
On 9 July 2024, a new amendment (EU) 2024/1860 to the MDR and IVDR was published, introducing, among other things, new extended transitional provisions for certain IVDs (legacy devices). This is the third amendment with extended transition timelines since 2017 (date when IVDR was published). Under this amendment, Class D devices have a new transition deadline of 31 December 2027 along with these requirements:
The timelines and obligations are specifically implemented in this third amendment (EU) 2024/1860, because previous amendments did not account for the workload associated with IVDR submissions. Many were submitted at the last minute (a few months before the deadline); Notified Bodies could not manage them and the risk of potential IVD shortages increased. The European Commission therefore discussed the potential impact for the smooth functioning of healthcare services and impacts on the EU market. Due to previous amendments, the amended regulation (EU) 2024/1860 gave manufacturers and Notified Bodies extra time to do their activities, but only with defined deadlines and limitations to apply for Notified Body capacities.
Put yourself in the position of a Notified Body: Notified Bodies need to account for their expected workload over an adequate timeline to support Class D manufacturers and manage the complex conformity process under IVDR. Therefore, Notified Bodies have communicated that only manufacturers who meet these deadlines will benefit from the longer IVDR transition timelines. A delay will mean the submissions will be added to the end of the review queue, thus facing the risk of delayed conformity assessment.
These deadlines for Class D IVD manufacturers start in May 2025 with the obligation to put in place an IVDR compliant QMS, lodge an IVDR-application with a Notified Body, and (in September 2025) sign a formal written agreement with a Notified Body. It is wise to adequately prepare and meet these timelines to make full use of the currently available Notified Body capacity.
We know how challenging and time-consuming a transition project to IVDR could be, especially if you are new to the required level of Technical Documentation and QMS. Good preparation and efficiency are important, but every manufacturer should realize that it is the time to act now if you want to keep your IVD devices on the EU market.
Pure Global offers tailored support, starting with the confirmation of your device class, up to Technical Documentation filing, QMS implementation, and Notified Body interaction. Learn more about our IVDR consulting services.
Explore our collection of articles, success stories, and regulatory updates, designed to help you take your product global.
Whether looking for more information or ready to partner with us, we're here to guide you through every step of the regulatory process.
Contact us