Pure Global Resource Center is our global database and AI powered regulatory tool. You can easily access global regulatory news, streamline document searches, compare clinical research data, and search through the databases with our AI and data powered research tools.



Explore our comprehensive database and tools to assist in your regulatory research. Discover key features designed to make your research process more efficient and effective.
Uncover regulatory access paths and product registration requirements with ease. Our data insights tools provide a one-stop search solution to streamline your compliance journey.
Support your regulatory team by receiving global regulatory updates and alerts to help manage your global registrations. Our regulation monitoring tools help keep your medical devices in compliance.
Discover the future of regulatory documentation with AI Digital Tools. Our software streamlines writing and offers seamless translation into over 25 languages, boosting compliance efficiency.
Our diverse databases provide efficient and comprehensive analysis of regulatory landscape expectations. Access past clinical trials data and registration details effortlessly.
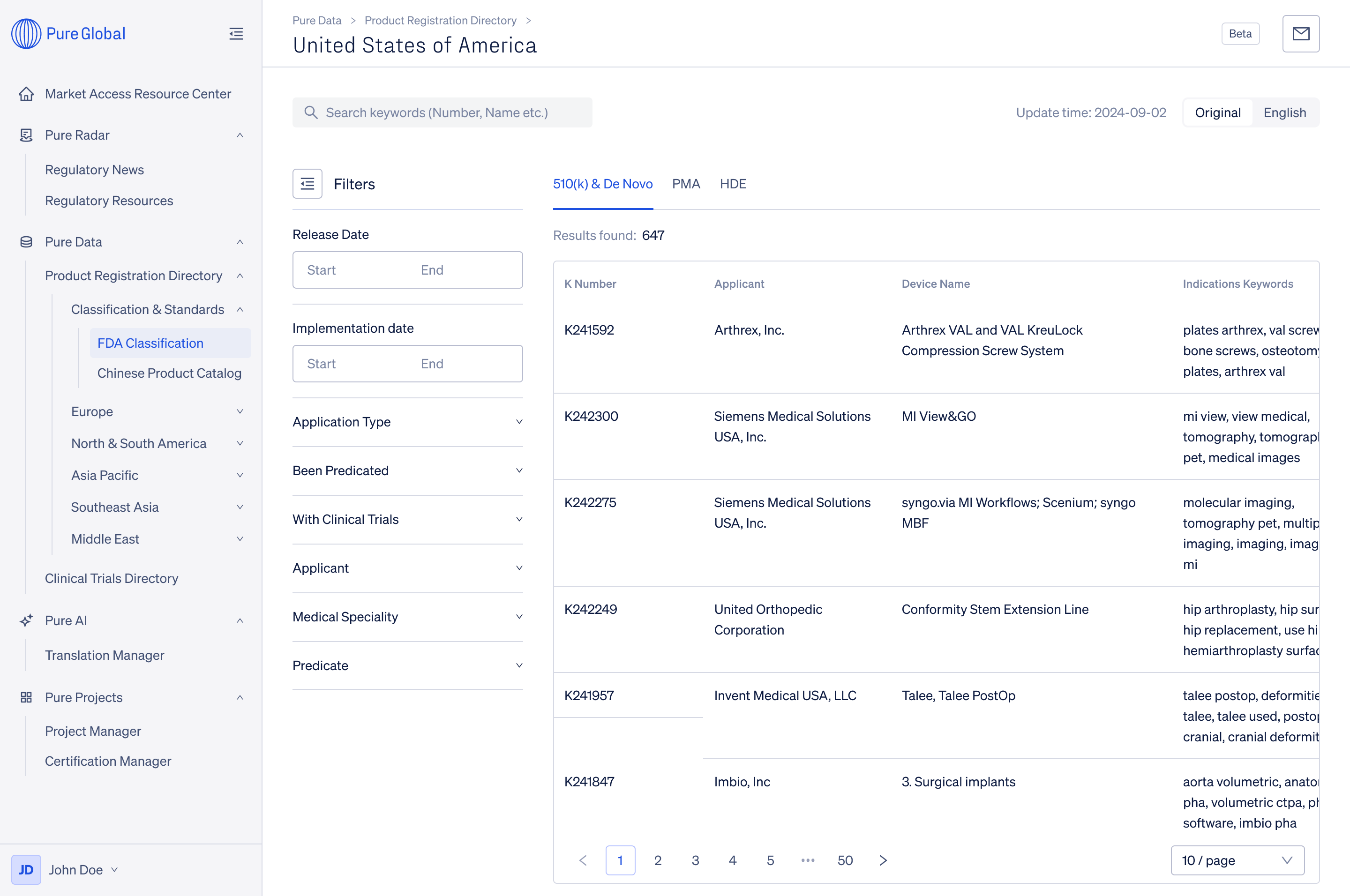
An invaluable tool for regulatory research, Product Registration Directory offers comprehensive registration information and effortless filtering. Access over 4 million registered medical device data from major countries worldwide.
A great asset for clinical research, our Clinical Trials Directory boasts over 1 million data entries, enabling seamless access to trial information including sites and sponsors. Discover over 200 thousand different related diseases with multiple study-stage records.

Navigate our database of regulatory updates and news to stay up to date with the latest regulations to ensure your device stays compliant.
Access the latest global medical device regulations. Browse and download original documents from worldwide regulatory bodies through our organized multinational access system with automatic translation across regions.

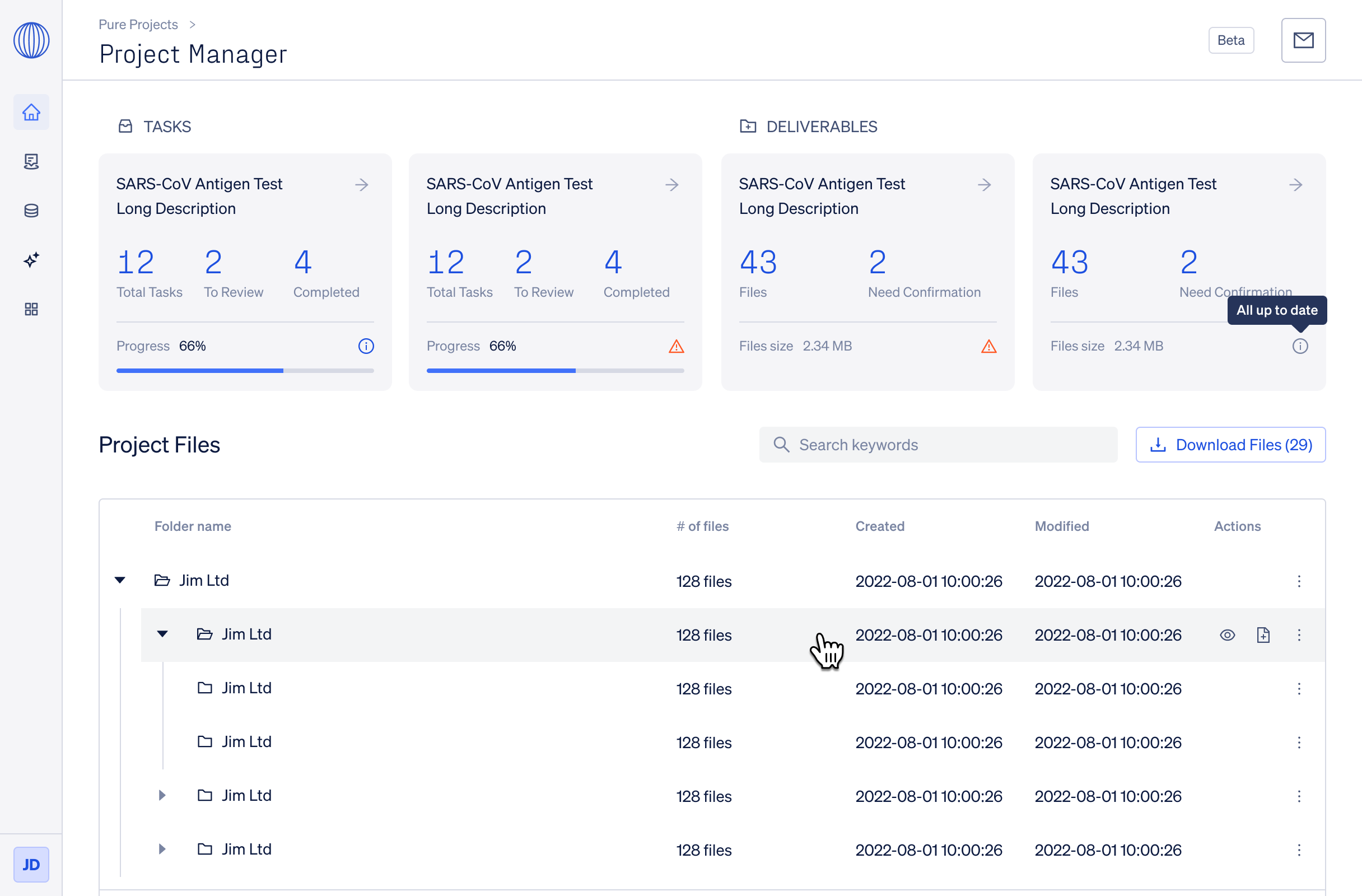
Utilize the Certification Manager to efficiently manage your registrations, receiving timely notifications for expiration or re-registration requirements, while effortlessly tracking all project-related information

Our advanced AI technology empowers users to efficiently handle technical documents, effectively reducing costs.
Our AI-generated Instructions for Use is the first of its kind in the industry. Using a large dataset of medical device instruction manuals, our AI has optimized large language models, enabling users to generate regulatory documents efficiently and quickly.

As companies expand into new markets, the need for product manuals in various languages arises. Our AI technology supports 25 languages, facilitating seamless translation of documents to meet diverse linguistic requirements.
