Unlocking Global Markets For MedTech Companies
We combine real-world experience with AI and data to build smart, efficient medical device regulatory consulting solutions for more than 30 markets.
global medical device regulatory consultants
With over 15 offices on five continents, Pure Global's team of regulatory experts provide real-time, ongoing regulatory support for medical device registrations and post-market compliance.
Reach Your Target Markets, Faster
We have the tools, knowledge, and experience to navigate the toughest regulatory challenges so you can start selling your device in your target markets.

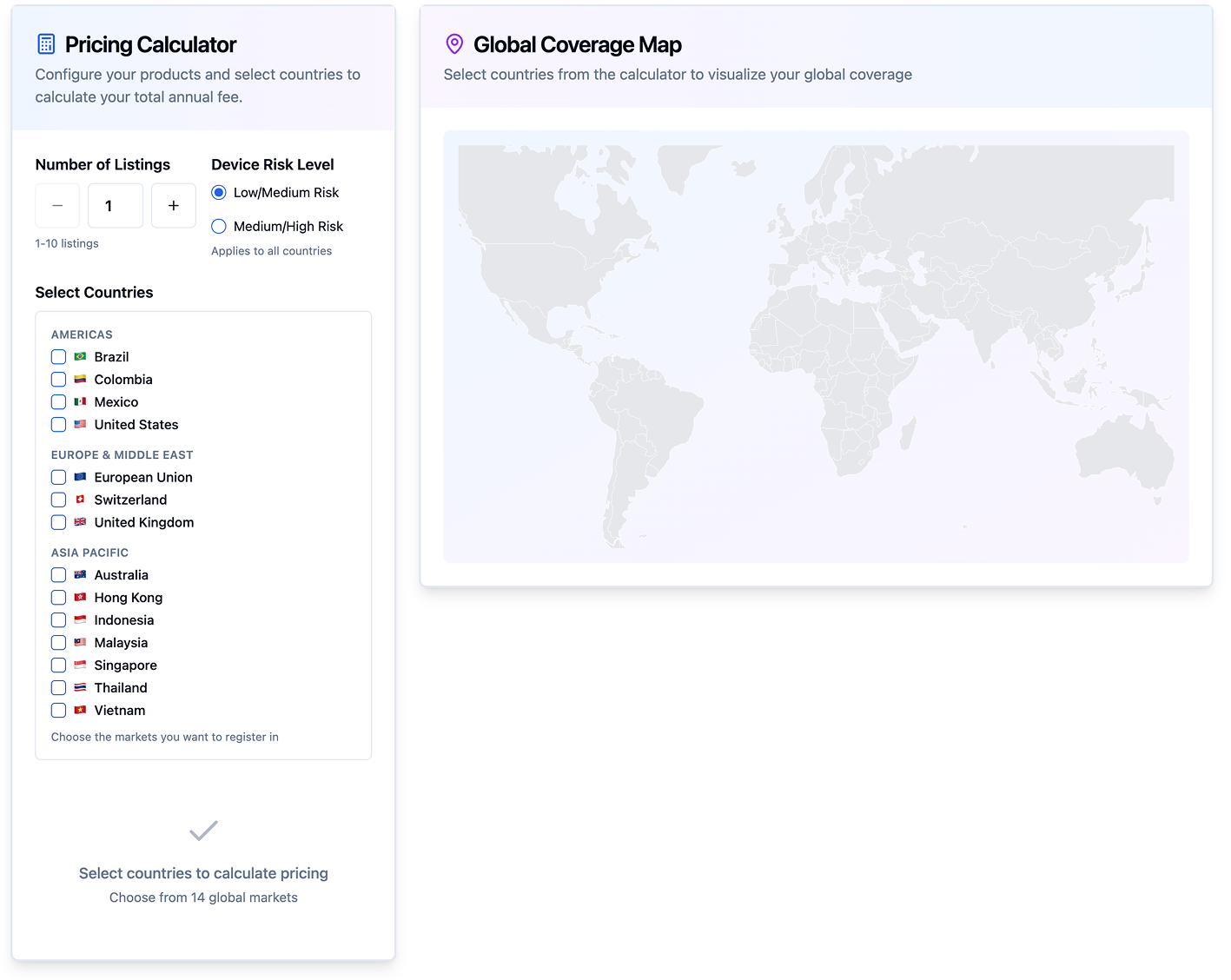
Simple, transparent pricing for global registration
Accelerate market entry with AI-powered dossier compilation and local representation, all for a flat annual fee. Starting at $2,000 USD per year for the first device (excludes government fees). Includes dossier submission, in-country representation, translation, modifications, distributor authorization, and post-market support.
Use our Fee Calculator to get your instant estimate.

Medical device regulatory consulting throughout the product lifecycle
From medical device regulatory compliance to entering new markets, our comprehensive solutions are designed for every challenge along the way.
Market Registration
We guide you through securing approvals and certifications from regulatory authorities, ensuring your device is ready for market entry.
Regulatory Services
We create efficient strategies for medical device regulatory approval, providing guidance on pathways and pre-submission activities.
Quality Assurance
We design processes and procedures that meet international quality standards and compliance requirements, both before and after market entry.
Local Representation
We establish our own operations in key markets worldwide, offering direct insight and support for local compliance and market entry.
AI & Data Tools
We develop AI- and data-powered research tools that aggregate global regulatory news, streamline document searches, and compare clinical data.
Other Services
We offer market analysis, lifecycle management, and targeted training to navigate global healthcare markets effectively.
Tailored solutions for medtech entrepreneurs and seasoned professionals
Pure Global provides scalable solutions to medical device and in vitro diagnostics manufacturers of all sizes to gain market access.

Startups
Get agile regulatory guidance and cost-effective solutions with our personalized support to quickly market your innovations.

Global Scaleups
Expand globally with our strategic support and partnership developments in emerging medical device and IVD markets.

Multinational Enterprise
Use our global regulatory strategies and tech integration, to navigate regulatory complexities and optimize your portfolio.
Latest Resources
Medical device regulatory updates, analysis, and success stories from our team of consultants, designed to take your product global.
Frequently
Asked Questions
What is the advantage of choosing Pure Global as my in-country representative over a commercial distributor?
Choosing a third party like Pure Global as your medical device regulatory in-country representative has numerous benefits:
Maintain control to your registration: In most markets, the in-country representative is the legal name on your registration. If your distributor is your representative, conflicts of interest can arise if you want to change or add distributors in that market.
Fulfill regulatory obligations: Your representative may have additional regulatory responsibilities to perform on your behalf, depending on the market. Distributors may not be informed about all the duties of an in-country representative or have the resources to perform them. Pure Global has deep experience in the regulatory requirements for all markets where we offer in-country representative services.
Expert regulatory guidance: Your in-country rep should guide you through the registration process, advise you on the completeness and formatting of your registration dossier, and be a proactive liaison with the local regulatory authority. It’s critical to select a regulatory expert who can advocate for your success in your target market.
How do I stay up to date on global regulatory changes?
Medical device compliance requirements are constantly evolving. Whether you’re preparing to enter a new market or maintaining existing authorizations, you must be proactive to stay informed of new regulatory developments.
Medical device regulatory consultants at Pure Global actively monitor and analyze developments in 30+ markets. Here’s how you can stay up-to-date:
- Weekly Regulatory News: A round-up of essential medical device regulatory news from around the world, published every week.
- Regulatory Blog: In-depth analysis of developments from our team of regulatory consultants.
- Join our community: Get insights from our expert team delivered to your inbox and the latest news from our AI-powered regulatory update tool.
What is the best stage to seek medical device regulatory consulting services?
As soon as possible. Many manufacturers wait until they receive a rejection or information request from a regulatory authority to bring in a regulatory consultant. But regulatory expertise can be invaluable as early as the device design and development stages. We can help you plan for the regulatory implications of your device’s intended use and indications for use, establish a holistic QMS that satisfies multiple market requirements, identify device-specific testing needs, and acquire compelling clinical data, if required. Early regulatory planning can save you time and money long term and facilitate your device’s entry to the market.
Can Pure Global support our entry to multiple markets?
Pure Global delivers end-to-end, on-the-ground in-country representative and medical device regulatory consulting services in the world’s biggest markets from our offices in the US, Europe, the UK, Asia, Latin America, and Australia. Through partnerships with local regulatory consulting firms, we offer extended market access to South Asia, the Middle East, Africa, and beyond.
Let's Talk,
Anywhere You Are.
Whether looking for more information or ready to partner with us, we're here to guide you through every step of the regulatory process.
Contact us

%201.svg)































