Join us for a series of videos and webinars focused on MedTech regulations, compliance, and innovation.

This video explains Philippines FDA (CDRRHR) rules for device classification, dossiers, labeling, and the critical role of a local representative.

This video explains the AMAR registration framework, U.S.-aligned device classifications, and the critical role of an Israeli Representative (IR).

This video explains SFDA’s medical device regulations, the role of an Authorized Representative (AR), and the steps foreign manufacturers must take to gain market access.

This video explains Mexico’s new Abbreviated Regulatory Pathway, launching September 1, 2025. It enables faster COFEPRIS approvals by recognizing prior authorizations from an expanded list of international regulators.

This video breaks down the MOH’s regulatory framework, device classification (Classes A–D), and the critical role of a Marketing Authorization Holder (MAH) in securing market access.

In this video, you will learn how foreign medical device manufacturers can register with the MHRA, achieve UKCA marking, and enter the UK’s medical device market.

Learn how to register medical devices in Thailand under the Thai FDA’s AMDD-aligned regulations, including risk-based classification, licensing requirements, and the mandatory role of a Local Authorized Representative.

This video explains how to register medical devices in Malaysia under the MDA’s risk-based system, covering classification, CAB reviews, and the critical role of a Local Authorized Representative.

In this video, you will learn how to register your medical devices in Hong Kong under the voluntary MDACS system managed by the MDD.

This video walks you through the full medical device registration process in Japan, from classification and QMS compliance to appointing a DMAH and submitting your application to PMDA.

Learn what manufacturers must do to legally market devices in Europe’s medtech market, from device classification and Notified Body assessment to EUDAMED registration and post-market surveillance.
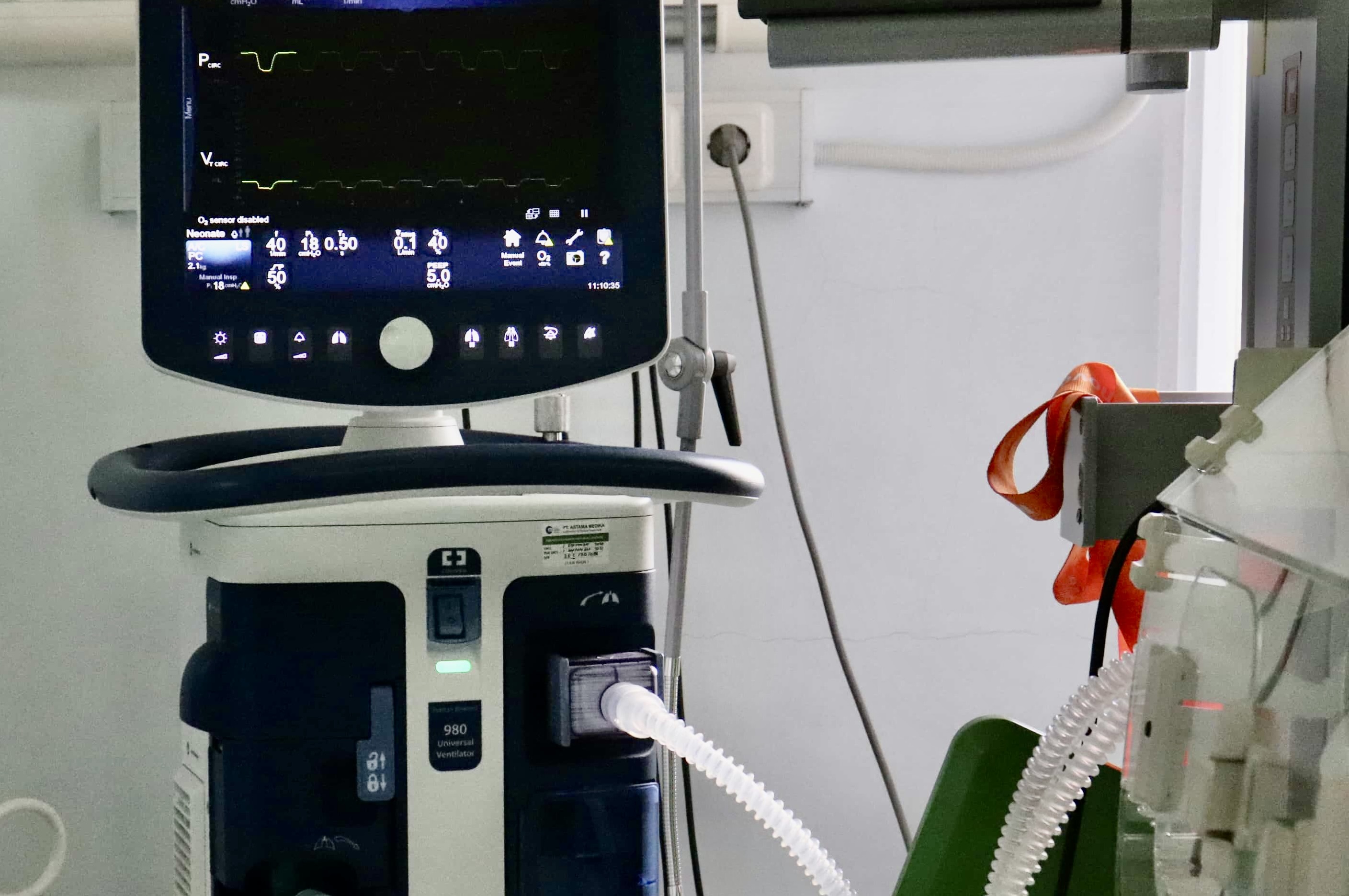
This video explains Brazil’s new UDI requirements under ANVISA and the upcoming compliance deadlines that start July 10, 2025 for Class IV devices.

Dr. Oliver Eikenberg explains the EU’s new MDCG 2025-5 guidance on IVD performance studies under the IVDR.

Learn how NADFC regulations, risk-based classifications, and the requirement for a licensed local representative shape market entry in Indonesia, and what documentation is essential for approval.
-min.jpg)
Learn about HSA’s four-tier classification system, key dossier and labeling requirements, and why appointing a local Registrant is essential for market entry and compliance.

This video explains the full process for registering a medical device in Canada with Health Canada, from risk classification and licensing requirements to MDSAP audits and post-market compliance.

This video walks you through the complete FDA registration process for medical devices in the United States, from classification and documentation to appointing a US Agent and submitting your application.

Discover how medical device companies can achieve sustainable global growth by integrating commercial planning with rigorous regulatory strategy.

This video walks you through the full medical device registration process in Colombia with INVIMA, including classification, documentation, and local representation requirements.

This video walks you through the full NMPA registration process for medical devices in China, including classification, dossier preparation, clinical trials, and local representation.

This video breaks down the full process for registering a medical device in Brazil with ANVISA, including classification, technical documentation, local representation, and post-market surveillance.

Learn how to register your medical device with the TGA and successfully enter the Australian market.

Everything manufacturers need to know to prepare for EUDAMED registration before the 2026 deadline.

Learn how to register a medical device in Mexico with COFEPRIS, from classification to approval, and unlock one of Latin America’s fastest-growing medtech markets.

In this recorded webinar, Dr. Oliver Eikenberg and Eva Camatini of IMQ, break down the most pressing challenges of IVDR compliance for IVD manufacturers.
Whether looking for more information or ready to partner with us, we're here to guide you through every step of the regulatory process.
Contact us